38 fda structured product labels
2011AA FDA Structured Product Labels Source Information IMPRINT_CODE. FDA Structured Product Label imprint attribute for code. 14314. ANDA. Abbreviated New (Generic) Drug application number for the MTHSPL drug. 11049. BLA. Therapeutic Biologic Applications number for the MTHSPL drug. UMLS Metathesaurus - MTHSPL (FDA Structured Product Labels) - Synopsis Authority The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site. Purpose
USFDA structured product labeling update - June 2020 July 12, 2020 admin 0. In month of June 2020, USFDA updated header of Structured Product Labeling (SPL) XML: Updated xml-stylesheet reference. Updated the schemaLocation of the urn:hl7-org:v3 namespace.
Fda structured product labels
Structured Product Labeling Validation Rules Guidance for Industry - Indexing Structured Product Labeling (Final) Guidance for Industry: Self-Identification of Generic Drug Facilities, Sites, and Organizations ... 4 Drug Labeling, Listing ... DailyMed - FDA Resources: SPL, Other Prescription Drug Labeling ... FDA's Structured Product Labeling Resources. Structured Product Labeling (SPL) is the standard format for electronic submission of the content of labeling. For SPL resources (including industry data standards for SPL), see FDA's SPL Resources page and the "Structured Product Labeling Resources" heading on FDA's Prescription Drug Labeling ... FDA Advisory No.2022-1163 || Public Health Warning Against the Purchase ... The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product, LABEL IS IN FOREIGN LANGUAGE. The abovementioned product was verified by FDA through postmarketing surveillance and shows no valid Certificate of Product Notification (CPN) as of 02 June 2022.
Fda structured product labels. DailyMed Sep 15, 2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products ... FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents.... 2010AA FDA Structured Product Labels Source Information Authority The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site. Purpose SPL Xforms | FDA To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ...
What is Structured Product Labeling (SPL)?, HL7, FDA, Regulatory ... Structured Product Labeling (SPL) is a standard document approved and issued by Health Level Seven (HL7) to exchange information related to product and facility. It is used as a base for Regulatory guidance document in exchange for product labeling content. SPL ensures control over critical product information that has led to a standard for product labeling. It is adopted by the Food and Drug ... FDA SPL - Structured Product & Drug Labeling Composition Process | Reed ... Structured product labeling for both prescription and over-the-counter (OTC) drugs must incorporate an overview of the scientific information needed for the correct and effective use of the drug. The labeling is broken up into sections including explanations for use (prescription drugs) or purpose (OTC drugs), adverse effects, and more. UMLS Metathesaurus - MTHSPL (FDA Structured Product Labels) - Metadata Metathesaurus FDA Structured Product Labels, 2022_02_25: Short Name: FDA Structured Product Labels: Family: MTHSPL: Metathesaurus Insertion Version: 2022AA: Restriction Level: 0: Language: ENG: Context Type: License Contact: RxNorm Customer Service U.S. National Library of Medicine 8600 Rockville Pike Bethesda MD United States Reed Tech | Best-In-Class Information-Based Solutions and ... Jun 17, 2022 · Build and submit UDI records electronically in Structured Product Labeling (SPL) format or seek subject-matter expertise. Manage Medical Device Product Data for UDI & Syndication Serving the medical device manufacturing industry in the areas of compliance, data management and regulatory requirements experience.
Structured Product Labeling (SPL) Terminology Files The NCIt-SPL terminology files provided here support the cooperative efforts of the Food and Drug Administration (FDA) and the National Cancer Institute's Thesaurus (NCIt) to develop terminology that facilitates the processing and review of SPL Terminology Files data. The efforts are described more fully on the Structured Products Labeling web ... Structured Product Labeling | Consumer Healthcare Products ... - CHPA In June 2009, the U.S. Food and Drug Administration (FDA) issued a guidance, "Providing Regulatory Submissions in Electronic Format — Drug Establishment Registration and Drug Listing" on its expanded requirements for submissions in the Structured Product Labeling (SPL) format. The FDA Amendments Act of 2007 (Public Law 110-85) requires ... Structured Product Labeling (SPL) | Data Conversion Laboratory Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard. MTHSPL (FDA Structured Product Labels) - Statistics FDA Structured Product Label imprint attribute for shape text: 18077: BLA: Therapeutic Biologic Applications number for the MTHSPL drug: 15324: NDA: New Drug Application number for MTHSPL drug: 11751: DCSA: Controlled Substance Act designation code (e.g. 0,2,3n) 7193: MARKETING_EFFECTIVE_TIME_HIGH:
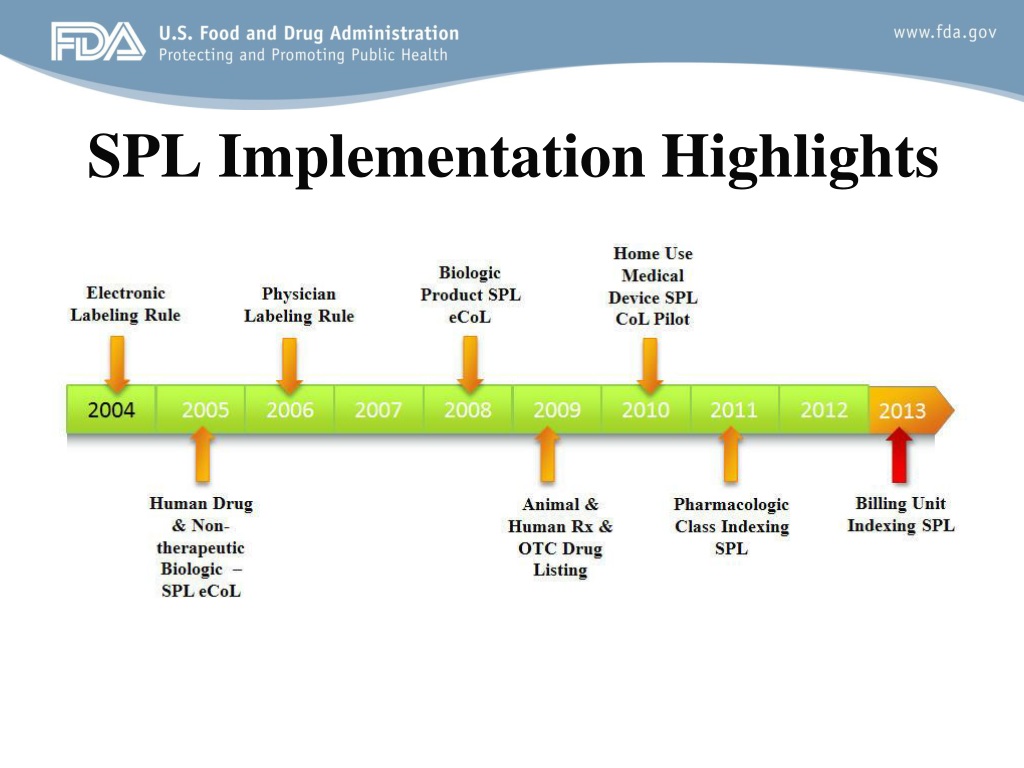
Structured Product Labeling Resources | FDA Apr 06, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.
2012AB FDA Structured Product Labels Source Information FDA Structured Product Label SET_ID code. 459423. NDC. National Drug Code corresponding to a clinical drug (e.g. 000023082503) 144687. DM_SPL_ID. DailyMed internal identifier for MTHSPL atom. 70092. LABELER.
MTHSPL (FDA Structured Product Labeling) Source Information AuthorityThe U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site.
Structured Product Labeling - Wikipedia Structured Product Labeling ( SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug.
DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.
NSDE | FDA Mar 31, 2022 · In this section: Structured Product Labeling Resources ... this file is generated from SPL documents sent to FDA for inclusion in the FDA Online Label Repository at labels.fda.gov. The information ...
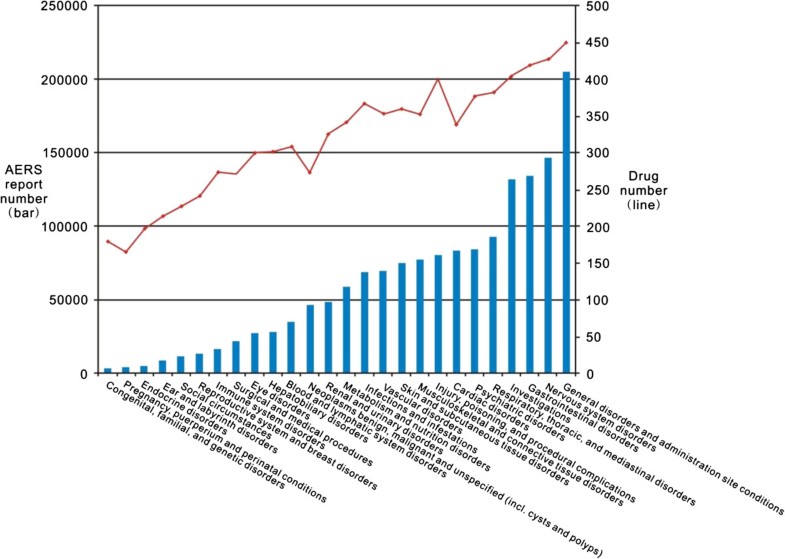
Assessing the Impact of HL7/FDA Structured Product Label (SPL) Content ... Methods. All 2217 labels available on DailyMed were downloaded as of February 23, 2007 and fed into an open-source HL7 v3 based data-infrastructure (HL7 JavaSIG). 12 The HL7 Reference Information Model (RIM) 13 is used directly as the object model. The HL7 RIM represents medicines, packages and ingredient-substances as (physical) Entities related to each other through Roles which specify the ...
Drug Labeling Overview - Food and Drug Administration Drug manufacturers and distributors submit documentation about their products to FDA in the Structured Product Labeling (SPL) format. The openFDA drug product labeling API returns data from this...
Structured Product Labeling - Food and Drug Administration Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. The openFDA...
IIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not currently being produced by the ...
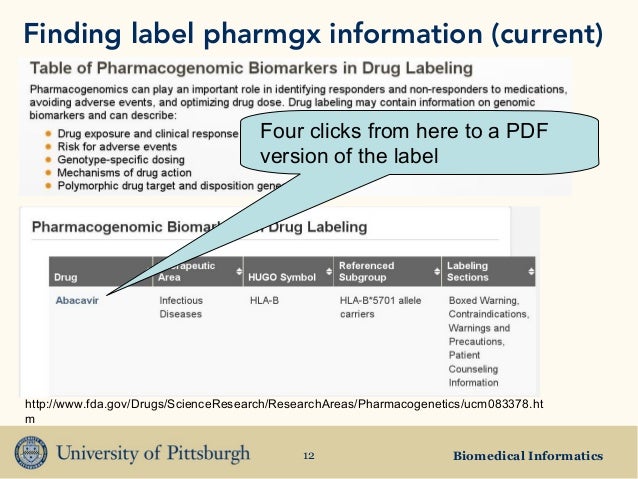
Indexing Structured Product Labeling | FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics...
Structured Product Labeling Resources - Food and Drug Administration The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL...
Introduction to FDA Structured Product Labeling - SPL R4 SPL. The Structured Product Labeling (SPL) specification is a document markup standard that specifies the structure and semantics for the regulatory requirements and content of the authorized published information that accompanies any medicine licensed by a national or international medicines licensing authority. UCUM.
FDA Label Search The device labeling has been reformatted to make it easier to read but its content has not been altered nor verified by FDA. The device labeling on this website may not be the labeling on currently...
FDA Advisory No.2022-1163 || Public Health Warning Against the Purchase ... The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product, LABEL IS IN FOREIGN LANGUAGE. The abovementioned product was verified by FDA through postmarketing surveillance and shows no valid Certificate of Product Notification (CPN) as of 02 June 2022.
DailyMed - FDA Resources: SPL, Other Prescription Drug Labeling ... FDA's Structured Product Labeling Resources. Structured Product Labeling (SPL) is the standard format for electronic submission of the content of labeling. For SPL resources (including industry data standards for SPL), see FDA's SPL Resources page and the "Structured Product Labeling Resources" heading on FDA's Prescription Drug Labeling ...
Structured Product Labeling Validation Rules Guidance for Industry - Indexing Structured Product Labeling (Final) Guidance for Industry: Self-Identification of Generic Drug Facilities, Sites, and Organizations ... 4 Drug Labeling, Listing ...











Post a Comment for "38 fda structured product labels"